Human MMP-9 DTSet enzyme-linked immunoassay kit
| Specification | 96*5 Test;96T*15 Test |
|---|---|
| Standard Curve Range | 31.25 pg/ml-2000 pg/ml |
| Standard Curve Gradient | 7 Points/3 Folds |
| Number of Incubations | 2 |
| Detectable sample | Liquid phase sample of soluble substances. For example: serum, plasma, cell culture supernatant, tissue grinding liquid, etc. |
| Sample Volume | 50 μl |
| Type | Not Ready-to-Use |
| Test Duration | 120min |
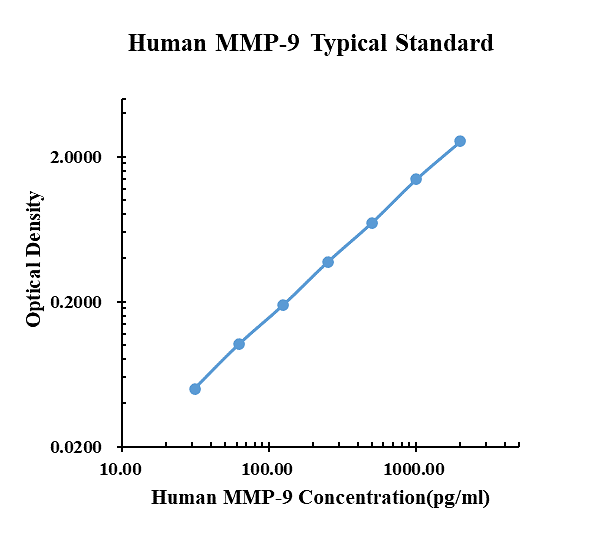
| pg/ml | O.D. | Average | Corrected | |
|---|---|---|---|---|
| 0.00 | 0.0595 | 0.0577 | 0.0586 | |
| 31.25 | 0.1056 | 0.1117 | 0.1087 | 0.0501 |
| 62.50 | 0.1595 | 0.1609 | 0.1602 | 0.1016 |
| 125.00 | 0.2484 | 0.2479 | 0.2482 | 0.1896 |
| 250.00 | 0.4347 | 0.4316 | 0.4332 | 0.3746 |
| 500.00 | 0.7562 | 0.7608 | 0.7585 | 0.6999 |
| 1000.00 | 1.4550 | 1.4530 | 1.4540 | 1.3954 |
| 2000.00 | 2.6010 | 2.6230 | 2.6120 | 2.5534 |
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DTSet Ancillary Reagent Kit (5 plates): containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and assay buffer.
- 96 well microplates: YOUKE Life, Catalog # DSEP01. Plate Sealers: YOUKE Life, Catalog # DSSF01.
- Coating Buffer: 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2-7.4, 0.2μm filtered . YOUKE Life, Catalog # DSCB01.
- Blocking Buffer: YOUKE Life, Catalog # DSBB01.
- Wash Buffer: 0.05% Tween® 20 in PBS, pH 7.2-7.4. YOUKE Life, Catalog # DSWB01.
- Assay Buffer: 0.5% BSA,0.05% Tween® 20,PBS Solution.YOUKE Life, Catalog # DSAB01
- Substrate Solution: Tetramethylbenzidine. YOUKE Life, Catalog # DSTS01.
- Stop Solution: 0.5mol/ml H2SO4. YOUKE Life, Catalog # DSSS01.
Product Data Sheet
Background: MMP-9
Matrix metalloproteinases (MMPs), also called matrixins, constitute a family of zinc and calcium dependent endopeptidases that function in the breakdown of the extracellular matrix (ECM) and in the processing of a variety of molecules in different subcellular environments. They play an important role in many normal physiological processes such as embryonic development, morphogenesis, reproduction, and tissue remodeling. They also participate in inflammatory and autoimmune disorders such as arthritis, cancer, and cardiovascular disease. While the amounts of newly synthesized MMPs are regulated mainly at the levels of transcription, the proteolytic activities of existing MMPs are controlled through both the activation of proenzymes or zymogens and the inhibition of active enzymes by endogenous inhibitors, alpha 2-Macroglobulin, and tissue inhibitors of metalloproteinases (TIMPs).
MMP-9 (also referred to as gelatinase B, 92 kDa type IV collagenase, 92 kDa gelatinase, and type V collagenase) is secreted as a glycosylated proenzyme. Activation of the proenzyme involves proteolytic removal of the N-terminal pro region, resulting in the 82 kDa active enzyme. Active human MMP-9 shares 72% and 74% amino acid sequence identity with mouse and rat MMP-9, respectively. In addition to the zinc-binding site, the catalytic domain also contains three contiguous fibronectin type II homology units responsible for binding gelatin. A proline-rich hinge region links the catalytic domain to the C-terminal hemopexin-like domain. In vitro treatment of the proenzyme with 4-aminophenylmercuric acetate (APMA) produces not only the active enzyme but also a C-terminal truncated form with activity comparable to that of the active form. MMP-9 degrades components of the ECM with high specific activity for denatured collagens (gelatin). It can cleave native collagens of type III, IV, V, and XI, as well as Elastin, Nidogen-1, and Vitronectin. MMP-9 can also cleave a variety of chemokines and growth factors (e.g. IL-1 beta, CXCL8/IL-8, CXCL7, CXCL4, CXCL1, Latent TGF-beta, membrane bound TNF-alpha, VEGF, and FGF basic), Amyloid beta peptide, Substance P, and Myelin Basic Protein. This action can increase or decrease the biological activity of soluble factors and can also liberate them from association with the ECM. MMP-9 can also trigger signaling through various transmembrane proteins or inhibit signaling by inducing their shedding from the cell surface (e.g. CD44, E-Cadherin, Integrins, ICAM-1, and IL-2 R alpha ).
MMP-9 is produced by a variety of normal and transformed cells including neutrophils, monocytes, macrophages, astrocytes, fibroblasts, osteoclasts, chondrocytes, keratinocytes, endothelial and epithelial cells. It exerts physiological and pathological angiogenic and remodeling effects on the vasculature. Activated neutrophils release proMMP-9 which is free of TIMP-1, allowing the liberation of pro-angiogenic FGF-2 from the ECM. MMP-9 in complex with TIMP-1 does not induce FGF-2 release. Neutrophil-derived MMP-9 exacerbates the inflammatory response, in part by generating collagen-derived peptides that induce the release of additional neutrophil MMP9. MMP-9 also plays a role in bone formation and remodeling, methamphetamineinduced behavioral sensitization and reward, the regulation of neuronal synapse remodeling, trophoblast invasion during implantation, and the inactivation of Serpin alpha 1-Proteinase Inhibitor. The shedding of adhesion proteins by MMP-9 has a direct effect on tumor cell invasiveness.
Circulating levels of MMP-9 are increased in many inflammatory disorders including intraluminal thrombus formation, atherosclerosis, Crohn's disease, hepatitis C virus infection, colorectal cancer, and Duchenne muscular dystrophy. The ratio of MMP-9 to TIMP-1 is also increased in multiple sclerosis serum and cystic fibrosis sputum, but it is decreased in the serum during cytomegalovirus infection. Levels of free MMP-9 and complexes of MMP-9 with Lipocalin-2/NGAL are elevated in the urine of ovarian cancer and uterine tract infection patients, respectively.

