Human IL-8/CXCL8 enzyme-linked immunoassay kit
| Specification | 96 Test |
|---|---|
| Sensitivity | 1.91 pg/ml (50 μl) ; 2.51 pg/ml (10 μl) |
| Standard Curve Range | 4.12~3000 pg/ml |
| Standard Curve Gradient | 7 Points/3 Folds |
| Number of Incubations | 2 |
| Detectable sample | Liquid phase sample of soluble substances. For example: serum, plasma, cell culture supernatant, tissue grinding liquid, etc. |
| Sample Volume | 50 μl/10 μl |
| Type | Fully Ready-to-Use |
| Operation Duration | 120min |
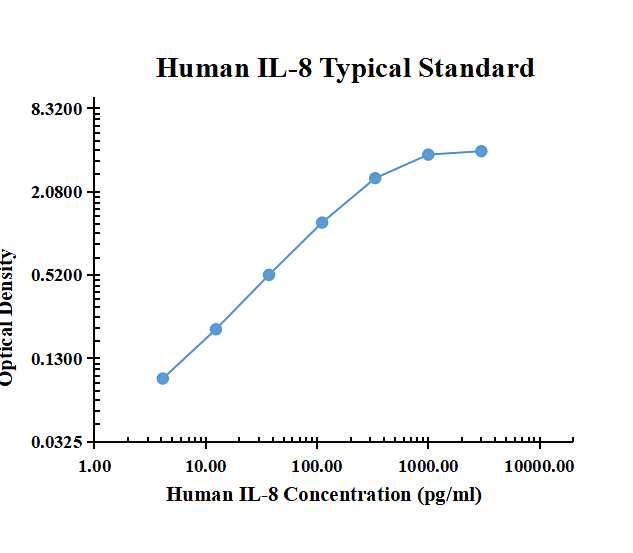
| pg/ml | O.D. | Average | Corrected | |
|---|---|---|---|---|
| 0.00 | 0.0712 | 0.0746 | 0.0729 | |
| 4.12 | 0.1577 | 0.1740 | 0.1659 | 0.0930 |
| 12.35 | 0.2702 | 0.2774 | 0.2738 | 0.2009 |
| 37.04 | 0.5827 | 0.6065 | 0.5946 | 0.5217 |
| 111.11 | 1.3060 | 1.3280 | 1.3170 | 1.2441 |
| 333.33 | 2.6770 | 2.6810 | 2.6790 | 2.6061 |
| 1000.00 | 3.8962 | 3.9586 | 3.9274 | 3.8545 |
| 3000.00 | 4.0852 | 4.2235 | 4.1544 | 4.0815 |
Precision
| Intra-assay Precision | Inter-assay Precision | |||||
| Sample Number | S1 | S2 | S3 | S1 | S2 | S3 |
| 22 | 22 | 22 | 6 | 6 | 6 | |
| Average(pg/ml) | 59.4 | 303.5 | 910.1 | 57.1 | 304.5 | 902.4 |
| Standard Deviation | 0.5 | 5.8 | 23.9 | 1.2 | 9.8 | 33.2 |
| Coefficient of Variation(%) | 2.9 | 5.6 | 4.7 | 3.5 | 4.3 | 5.1 |
Intra-assay Precision (Precision within an assay) Three samples of known concentration were tested twenty times on one plate to assess intra-assay precision.
Inter-assay Precision (Precision between assays) Three samples of known concentration were tested six times on one plate to assess intra-assay precision.
Spike Recovery
The spike recovery was evaluated by spiking 3 levels of human IL-8 into health human serum sample. The un-spiked serum was used as blank in this experiment.
The recovery ranged from 88% to 112% with an overall mean recovery of 97%.
Sample Values
| Sample Matrix | Sample Evaluated | Range (pg/ml) | Detectable (%) | Mean of Detectable (pg/ml) |
|---|---|---|---|---|
| Serum | 30 | 113.36- 1751.50 | 100 | 741.07 |
Serum/Plasma – Thirty samples from apparently healthy volunteers were evaluated for the presence of IL-8 in this assay. No medical histories were available for the donors.
Product Data Sheet
Background: IL-8/CXCL8
Interleukin-8 (IL-8), also known as IL-8, GCP-1, and NAP-1, is a heparin-binding 8-9 kDa member of the alpha, or CXC family of chemokines. There are at least 15 human CXC family members that all adopt a three beta -sheet/one alpha -helix structure. Most CXC chemokines show an N-terminal Glu-Leu-Arg (ELR) tripeptide motif. IL-8 circulates as a monomer, homodimer, and heterodimer with CXCL4/PF4. The monomer is considered the most bio-active, while the heterodimer can potentiate PF4 activity. IL-8 oligomerization is modulated by its interactions with matrix and cell surface glycosaminoglycans (GAGs). Mature human IL-8 shares 65-69% amino acid (aa) identity with canine, feline, and porcine IL-8. There is no IL-8 gene counterpart in rodent.
Multiple isoforms of IL-8 are generated through both alternative splicing and differential proteolytic cleavage. In humans, alternative splicing generates an iso-form with an eleven aa substitution at the C-terminus. Proteolytic processing results in N-terminal truncation of IL-8 and is likely a cell-specific event. For example, fibroblasts and endothelial cells generate the 1-77 form by cleaving IL-8 following Glu21, while monocytes and lymphocytes generate the 6-77 form by cleaving following Leu25. These truncated forms generally show increased bioactivity, particularly through the CXCR1 receptor. IL-8 can also undergo citrullination on Arg27 of the precursor, a modification that increases its half-life and ability to induce leukocytosis. A wide variety of cells secrete IL-8 including monocytes and neutrophils, fibroblasts and keratinocytes, mast cells, visceral smooth muscle cells, dendritic cells, type II great alveolar cells, and endothelial cells.
IL-8 bioactivity is mediated through two G-protein-coupled receptors, termed CXCR1/IL-8 RA and CXCR2/IL-8 RB. CXCR1 is 45-50 kDa in size and is used almost exclusively by IL-8. CXCR2 is 35-40 kDa in size and is used by nearly all CXC chemokines. Both CXCR1 and CXCR2 constitutively associate into functional homodimers. They can also heterodimerize, but these complexes dissociate following IL-8 binding. CXCR2 responds to low concentrations of IL-8 and is principally associated with chemotaxis and MMP-9 release. CXCR1, in contrast, responds to high concentrations of IL-8 and is associated with respiratory burst and phospholipase D2 activation. Thus, CXCR2 ligation induces leukocyte adhesion to activated vascular endothelium and migration to sites of inflammation, while CXCR1 ligation primes neutrophil antimicrobial activity. IL-8 can also form a complex with Serpin A1/alpha-1 Antitrypsin, and this prevents IL-8 interaction with CXCR1.
In addition to its pro-inflammatory effects, IL-8 is involved in angiogenesis and the pathogenesis of atherosclerosis and cancer. It induces VEGF expression in vascular endothelial cells and functions as an autocrine factor for EC growth and angiogenesis. It is upregulated in atherosclerotic lesions and is elevated in the serum and cerebrospinal fluid following myocardial infarction. In cancer, IL-8 promotes epithelial-mesenchymal transition as well as tumor cell invasiveness and metastasis.

