Mouse IL-1β enzyme-linked immunoassay kit
| Specification | 96 Test |
|---|---|
| Sensitivity | 0.91 pg/ml (10 μl) |
| Standard Curve Range | 15.63~1000 pg/ml |
| Standard Curve Gradient | 7 Points |
| Number of Incubations | 2 |
| Detectable sample | Liquid phase sample of soluble substances. For example: serum, plasma, cell culture supernatant, tissue grinding liquid, etc. |
| Sample Volume | 10 μl |
| Type | Fully Ready-to-Use |
| Operation Duration | 120min |
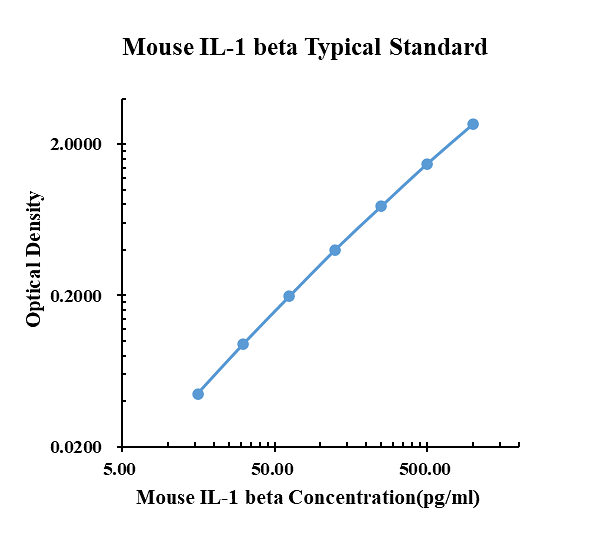
| pg/ml | O.D. | Average | Corrected | |
|---|---|---|---|---|
| 0.00 | 0.0651 | 0.0701 | 0.0676 | |
| 15.63 | 0.1104 | 0.1143 | 0.1124 | 0.0448 |
| 31.25 | 0.1660 | 0.1609 | 0.1635 | 0.0959 |
| 62.50 | 0.2623 | 0.2676 | 0.2650 | 0.1974 |
| 125.00 | 0.4740 | 0.4650 | 0.4695 | 0.4019 |
| 250.00 | 0.8593 | 0.8316 | 0.8455 | 0.7779 |
| 500.00 | 1.5070 | 1.6060 | 1.5565 | 1.4889 |
| 1000.00 | 2.7780 | 2.8080 | 2.7930 | 2.7254 |
Precision
| Intra-assay Precision | Inter-assay Precision | |||||
| Sample Number | S1 | S2 | S3 | S1 | S2 | S3 |
| 22 | 22 | 22 | 6 | 6 | 6 | |
| Average(pg/ml) | 18.9 | 93.0 | 290.2 | 17.4 | 97.5 | 280.7 |
| Standard Deviation | 1.0 | 4.5 | 16.9 | 2.3 | 8.5 | 14.6 |
| Coefficient of Variation(%) | 5.3 | 4.9 | 5.8 | 6.1 | 6.1 | 5.7 |
Intra-assay Precision (Precision within an assay) Three samples of known concentration were tested twenty times on one plate to assess intra-assay precision.
Inter-assay Precision (Precision between assays) Three samples of known concentration were tested six times on one plate to assess intra-assay precision.
Spike Recovery
The spike recovery was evaluated by spiking 3 levels of mouse IL-1 beta into health mouse serum sample. The un-spiked serum was used as blank in this experiment.
The recovery ranged from 81% to 111% with an overall mean recovery of 98%.
Sample Values
| Sample Matrix | Sample Evaluated | Range (pg/ml) | Detectable (%) | Mean of Detectable (pg/ml) |
|---|---|---|---|---|
| Serum | 30 | n.d.-3.66 | 13 | 1.08 |
Serum/Plasma – Thirty samples from apparently healthy mice were evaluated for the presence of IL-1β in this assay. No medical histories were available for the donors. n.d. = non-detectable. Samples measured below the sensitivity are considered to be non-detectable.
Product Data Sheet
Background: IL-1β
The Interleukin 1 (IL-1) family of proteins consists of IL-1 alpha, IL-1 beta, and the IL-1 receptor antagonist (IL-1ra). IL-1 alpha and IL-1 beta bind to the same cell surface receptors and share biological functions. IL-1 is not produced by unstimulated cells of healthy individuals with the exception of skin keratinocytes, some epithelial cells, and certain cells of the central nervous system. However, in response to inflammatory agents, infections, or microbial endotoxins, a dramatic increase in the production of IL-1 by macrophages and various other cell types is seen. IL-1 beta plays a central role in immune and inflammatory responses, bone remodeling, fever, carbohydrate metabolism, and GH/IGF-I physiology. Inappropriate or prolonged production of IL-1 has been implicated in a variety of pathological conditions including sepsis, rheumatoid arthritis, inflammatory bowel disease, acute and chronic myelogenous leukemia, insulindependent diabetes mellitus, atherosclerosis, neuronal injury, and aging-related diseases.
IL-1 alpha and IL-1 beta are structurally related polypeptides that show approximately 25% homology at the amino acid (aa) level. Both are synthesized as 31 kDa precursors that are subsequently cleaved into mature proteins of approximately 17.5 kDa. Cleavage of the IL-1 beta precursor by Caspase-1/ICE is a key step in the inflammatory response. Neither IL-1 alpha nor IL-1 beta contains a typical hydrophobic signal peptide, but evidence suggests that these factors can be secreted by non-classical pathways. A portion of unprocessed IL-1 alpha can be presented on the cell membrane and may retain biological activity. The precursor form of IL-1 beta, unlike the IL-1 alpha precursor, shows little or no biological activity in comparison to the processed form. Both unprocessed and mature forms of IL-1 beta are exported from the cell.
IL-1 alpha and IL-1 beta exert their effects through immunoglobulin superfamily receptors that additionally bind IL-1ra. The 80 kDa transmembrane type I receptor (IL-1 RI) is expressed on T cells, fibroblasts, keratinocytes, endothelial cells, synovial lining cells, chondrocytes, and hepatocytes. The 68 kDa transmembrane type II receptor (IL-1 RII) is expressed on B cells, neutrophils, and bone marrow cells. The two IL-1 receptor types show approximately 28% homology in their extracellular domains but differ significantly in that the type II receptor has a cytoplasmic domain of only 29 aa, whereas the type I receptor has a 213 aa cytoplasmic domain. IL-1 RII does not appear to signal in response to IL-1 and may function as a decoy receptor that attenuates IL-1 function. The IL-1 receptor accessory protein (IL-1 RAcP) associates with IL-1 RI and is required for IL-1 RI signal transduction. IL-1ra is a secreted molecule that functions as a competitive inhibitor of IL-1. Soluble forms of both IL-1 RI and IL-1 RII have been detected in human plasma, synovial fluids, and the conditioned media of several human cell lines. In addition, IL-1 binding proteins that resemble soluble IL-1 RII are encoded by vaccinia and cowpox viruses.

